Oil additives-antioxidant-Control of sediment production
Time:
2025-07-21
Lube and lubricant additives

The quality of lubricity is inseparable from the quality of oil additives. Lubricating oil additives are the main way to improve the quality of lubricating oil and expand the types of lubricating oil. They are also an important way to improve lubricating oil performance, save energy, and reduce environmental pollution. CheMsot has been engaged in the engine oil additives industry in China for many years and has a certain understanding of the mechanism of action, product application, development trend and market environment of oil additive. CheMost hopes to share its own experience and knowledge here. We will introduce Chinese lubricating oil additives in detail and combine them with high-quality lubricating oil additives from all over the world.
With the increasing demand for energy conservation and environmental protection in various countries and regions in recent years, under the background of global carbon credit policies, the mechanical equipment used in industry is gradually becoming smaller in size, lighter in weight, and more powerful. As a result, more stringent requirements are put forward for the lubricants and additives used in mechanical equipment. The base oil used in lubricating oil is also gradually developing from mineral oil to synthetic oil and vegetable oil.
In addition to improving the performance of lube, increasing the service life of lubricants is also an important way to reduce environmental pollution. In addition, after lubricants have good antioxidant properties, their performance will also be improved accordingly. We will start with antioxidants. When it comes to antioxidants, we should first know why we need to improve antioxidant capacity? Before fully understanding the oxidation mechanism of hydrocarbons, lubricant experts gradually realized that some oils have better antioxidant properties. This difference was eventually confirmed to be due to the existence of natural antioxidants. People found that some natural antioxidants contain sulfur or complex functional groups. Therefore, it is not surprising that some additives that give oils special properties, such as sulfur-containing chemicals, can provide additional antioxidant properties. In addition to sulfur-containing additives that can provide oxidation stability, people have found that phenol also has the same properties, which has promoted the development of thiophenol. Some amines and metal phosphides or sulfides have also been proven to provide oxidation stability.
One of the main reasons why lubricants lose their effectiveness is oxidative degradation. Therefore, at least one antioxidant is added to lubricants currently produced to improve their oxidative stability or enhance other properties. One of the most important aspects of lubricants is that they require good oxidative stability. Substances produced after lubricant oxidation not only reduce its performance, but also shorten its service life, and also cause wear to the lubricated parts used. Hydrocarbons exposed to oxygen and heat will accelerate the oxidation process, especially in the presence of metals such as iron and copper, the oil is more susceptible to oxidation. For example, the heat generated by the friction of metal parts in an internal combustion engine makes the inside of the internal combustion engine a chemical reactor that promotes the oxidation process, so engine lubricants may be more susceptible to oxidation than other lubricants. However, any lubricant exposed to air and heat will eventually oxidize.
At present, many effective antioxidant additives have been developed and are widely used in engine oils, automatic transmission fluids, gear oils, turbine oils, compressor oils, greases, hydraulic fluids and metalworking fluids. The oil-soluble organic compounds and organometallic antioxidants currently used mainly include the following categories:
1. Sulfur compounds
2. Sulfur-nitrogen compounds
3. Phosphorus compounds
4. Sulfur-phosphorus compounds
6. Boron compounds
Oxidation mechanisms and antioxidant effects
Before introducing these antioxidants in detail, we must first understand what oxidation is and what antioxidant effects are.
It is currently known that the oxidation process of hydrocarbon lubricants is a self-oxidation process. Oxidation increases the acid value and viscosity of lubricants. More importantly, insoluble deposits and varnishes may be generated, resulting in increased engine wear, lubricity, reduced fuel economy, etc. Self-oxidation is a chain reaction of free radicals, including four different reaction stages: chain initiation, chain growth, chain branching, and chain termination.
The chain initiation stage occurs when hydrocarbon substances form hydrocarbon free radicals due to dehydrogenation and C-C bond cleavage in the energy environment of oxygen, heat, ultraviolet light or mechanical shear stress.  The strength of the C-H bond in the hydrocarbon molecule and the stability of the formed free radical determine the order of strength of the R-H bond homolysis to form free radicals as follows: phenyl < methyl < methylene < methylene < tertiary group < benzyl group. Therefore, hydrogen on the tertiary group or the carbon-carbon double bond and the hydrogen on the α
The strength of the C-H bond in the hydrocarbon molecule and the stability of the formed free radical determine the order of strength of the R-H bond homolysis to form free radicals as follows: phenyl < methyl < methylene < methylene < tertiary group < benzyl group. Therefore, hydrogen on the tertiary group or the carbon-carbon double bond and the hydrogen on the α  position of the aromatic ring are prone to homolysis. Chain initiation will proceed very slowly under normal conditions, but it will occur quickly under conditions of increased temperature or the presence of metal catalysts (such as copper, iron, nickel, vanadium, manganese, cobalt, etc.).
position of the aromatic ring are prone to homolysis. Chain initiation will proceed very slowly under normal conditions, but it will occur quickly under conditions of increased temperature or the presence of metal catalysts (such as copper, iron, nickel, vanadium, manganese, cobalt, etc.).
The first stage of chain growth is the formation of alkyl peroxyl radicals (ROO·) by alkyl radicals and oxygen, which is an irreversible reaction.
The reaction rate of alkyl radicals with oxygen is extremely fast, and its specific rate depends on the substituent of the free radical. Once the alkyl peroxyl radical is formed, it can randomly capture hydrogen from the hydrocarbon molecule to generate hydrocarbon hydroperoxide (ROOH) and a new alkyl radical (R·). B
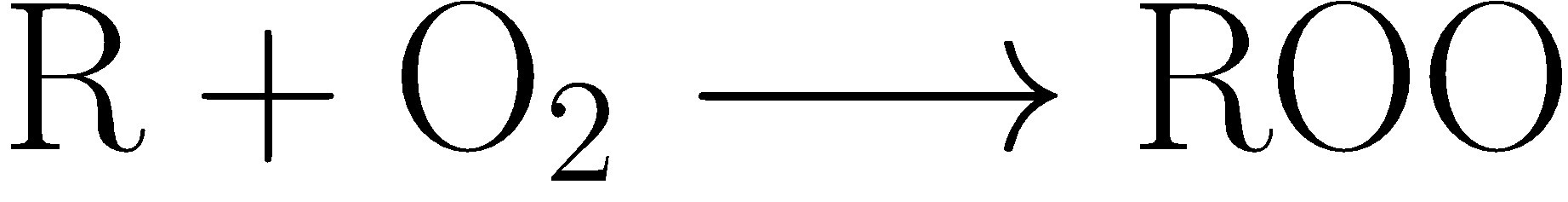
ased on this mechanism, a large number of hydrocarbon molecules will be oxidized to alkyl peroxy radicals every time alkyl radicals are formed.
The chain branching stage begins with the cleavage of hydroperoxides into alkoxy radicals (RO·) and hydroxyl radicals (HO·). This reaction requires a lot of activation energy, and only when the temperature is higher than 150°C will there be a significant reaction. The presence of catalytic metal ions will accelerate this process.  The formation of free radicals will undergo some possible reactions. The chain branching reaction is a critical step. As the degree of oxidation continues, not only a large number of alkoxy radicals will be generated to accelerate oxidation, but also low molecular weight aldehydes and ketones will be generated, which affect many physical properties of lubricants,
The formation of free radicals will undergo some possible reactions. The chain branching reaction is a critical step. As the degree of oxidation continues, not only a large number of alkoxy radicals will be generated to accelerate oxidation, but also low molecular weight aldehydes and ketones will be generated, which affect many physical properties of lubricants,
such as quickly reducing the viscosity of oil products, increasing oil evaporation losses and increasing polarity. Under high temperature oxidation conditions, aldehydes and ketones condense through acid-catalyzed alcohol-aldehyde condensation reactions. The condensation products can lead to the formation of polymer degradation products, which eventually manifest as sludge and paint film deposits in the engine.
Chain termination is the increase in viscosity of oil products due to the increase in high molecular weight hydrocarbons as the degree of oxidation deepens. When the viscosity of oil products reaches a certain level, the diffusion of oxygen is at a limited level, and the chain termination reaction takes the leading role.  Two alkyl radicals can combine to form a hydrocarbon molecule, or an alkyl radical and an alkyl peroxy radical can combine to form a peroxide molecule. However, this peroxide molecule is very unstable and will produce more alkyl peroxy radicals. In the process of chain termination, the formation of carbonyl compounds and alcohols also includes peroxy radicals containing α-hydrogen atoms.
Two alkyl radicals can combine to form a hydrocarbon molecule, or an alkyl radical and an alkyl peroxy radical can combine to form a peroxide molecule. However, this peroxide molecule is very unstable and will produce more alkyl peroxy radicals. In the process of chain termination, the formation of carbonyl compounds and alcohols also includes peroxy radicals containing α-hydrogen atoms.
Now we have a general understanding of the oxidation process, and after understanding the oxidation process, we also have a general idea of the anti-oxidation effect, which we will share later.
MORE BLOG
CheMost
CheMost Additives CO.,LTD
ADDRESS: CheMost Additives CO.,LTD, Jinzhou city, Liaoning provice, China
To learn more about CheMost, please click the button to contact us anytime.
Get product catalogCopyright© 2025 CheMost Additives CO.,LTD
Website:300.cn jinzhou.300.cn | SEO | Privacy Policy






